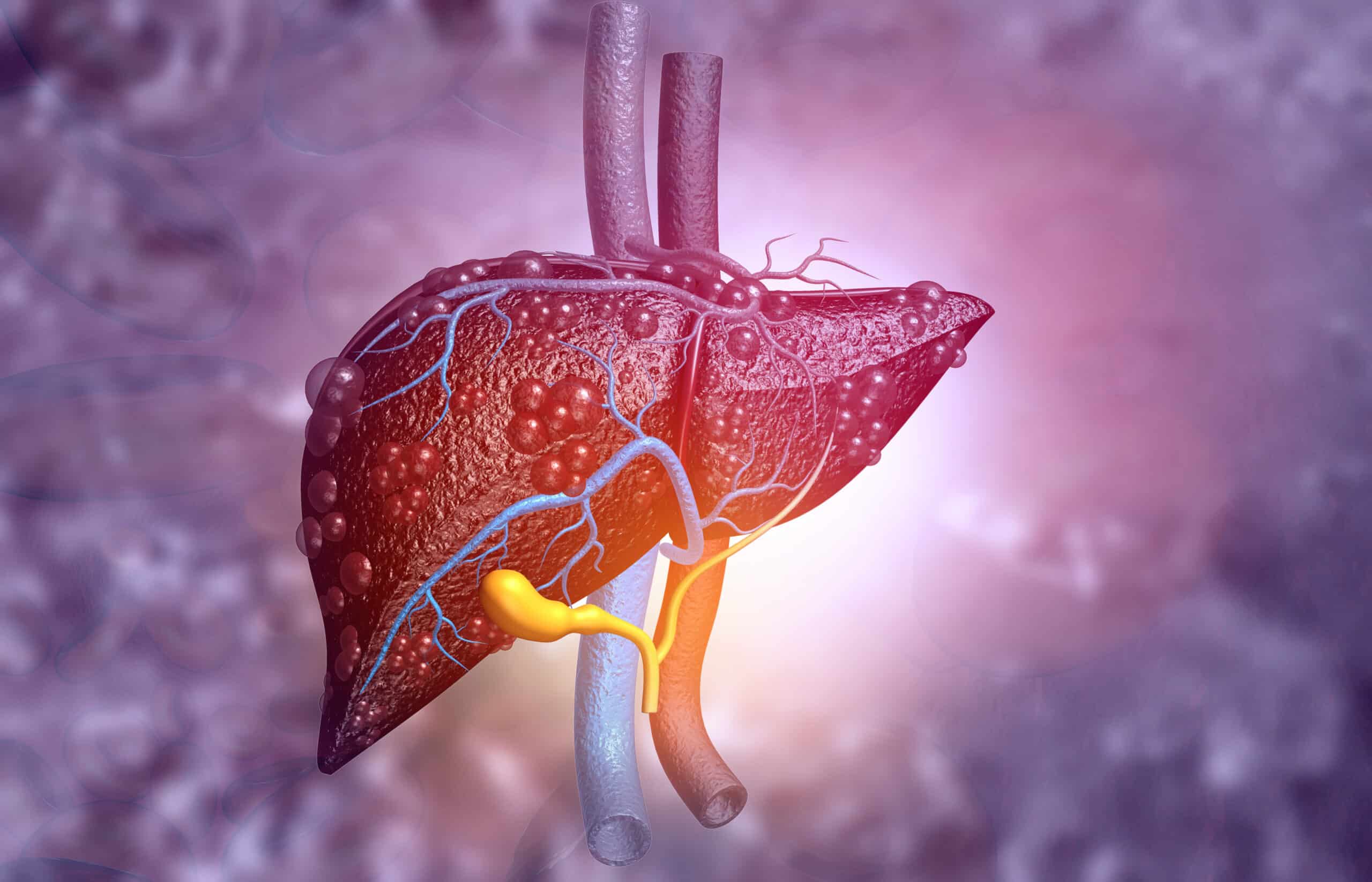
The US Food and Drug Administration has approved injectable semaglutide (Wegovy) for adults with metabolic dysfunction-associated steatohepatitis (MASH) who have moderate to advanced liver fibrosis without cirrhosis.
Major Clinical Breakthrough
MASH affects 6% of US adults and represents a serious progression of fatty liver disease that can lead to:
- Advanced liver scarring
- Liver cancer
- Need for liver transplant
- Cardiovascular complications
Phase 3 Trial Results
The FDA approval was based on compelling clinical data showing:
- 63% of participants achieved steatohepatitis resolution with weekly Wegovy injections
- 34% placebo response rate in comparison group
- 18-month treatment period demonstrated significant efficacy
- Ongoing monitoring for long-term health improvements
Expanded Treatment Options
Wegovy (semaglutide), a GLP-1 receptor agonist manufactured by Novo Nordisk, was previously approved for:
- Adult obesity treatment
- Overweight patients with comorbidities
- High-risk cardiovascular patients to prevent heart attacks
This MASH indication represents the first GLP-1 therapy specifically approved for severe liver disease, offering new hope for patients with this progressive condition.
For MASH evaluation and GLP-1 treatment consultation, contact Rutherford Medical Center at (770) 696-2265